
The more active metal forms a new compound containing metal cations. In general, the activity of a metal may be defined as follows:Īn active metal will react with a compound of a less active metal, which is converted to its “free element” form. A more active metal always replaces the ion of a less active metal. Single replacement reactions will occur spontaneously in one direction only (compare Equations 1 and 2). The reaction of aluminum with copper (II) chloride (Equation 1) is classified as a single replacement reaction – aluminum reacts with and “replaces” copper ions in copper (II) chloride. Consider equation 1 and 2 below:ĢAl (s) + 3CuCl 2(aq) -> 2AlCl 3(aq) + 3Cu (s) (Equation 1)Ĭu (s) + AlCl 3(aq) -> No Reaction (Equation 2) To determine the activity of metals you can compare the reactions of metals with different How can we determine the relative reactivity of different metals? Gold is a highly valuable jewelry metal because it is essentially unreactive. Copper is used in electrical wiring because it conducts electricity extremely well and resists corrosion better than many metals. Although iron is the most common metal used in manufacturing, it must be protected againstĬorrosion because rusts easily. The total energy for the process is the sum of the energies of the individual steps.The usefulness of metals in structural and other applications depends on their physical and chemical.Write the net balanced equation for this reaction that includes all of the steps above.second ionization energy (ga s) (+1733):.first ionization energy (gas) (+906 kJ/mol):.For zinc, write the chemical equations for the individual steps:.There are several steps to get from metal in the solid state to Zn 2+(aq). So this is really more than the loss of electrons. Zinc is defined as reacting vigorously with H+ in aqueous solution. Write the chemical equation for the oxidation of Zn.Since the experimental data indicates that zinc metal reacts vigorously with acid, there must be more to the activity of zinc than simple periodic trends.
#Reactivity series practice series#

What is the oxidizing agent? What is the reducing agent?.Which species is oxidized in this reaction? Which is reduced?.A typical activity series reaction is the following: So, if one metal is going to lose its electrons, another species (a metal ion) has to pick them up. Metals (in their elemental form) don’t react with each other. Predict which is more active, Rb or Cs? Explain.Predict which is more active, Rb or Sr? Why?.Is this an oxidation or a reduction? Explain.

Chemists are interested in trying to find the best metals to utilize.Īctivity is the tendency of a metal (and hydrogen) to LOSE electrons.
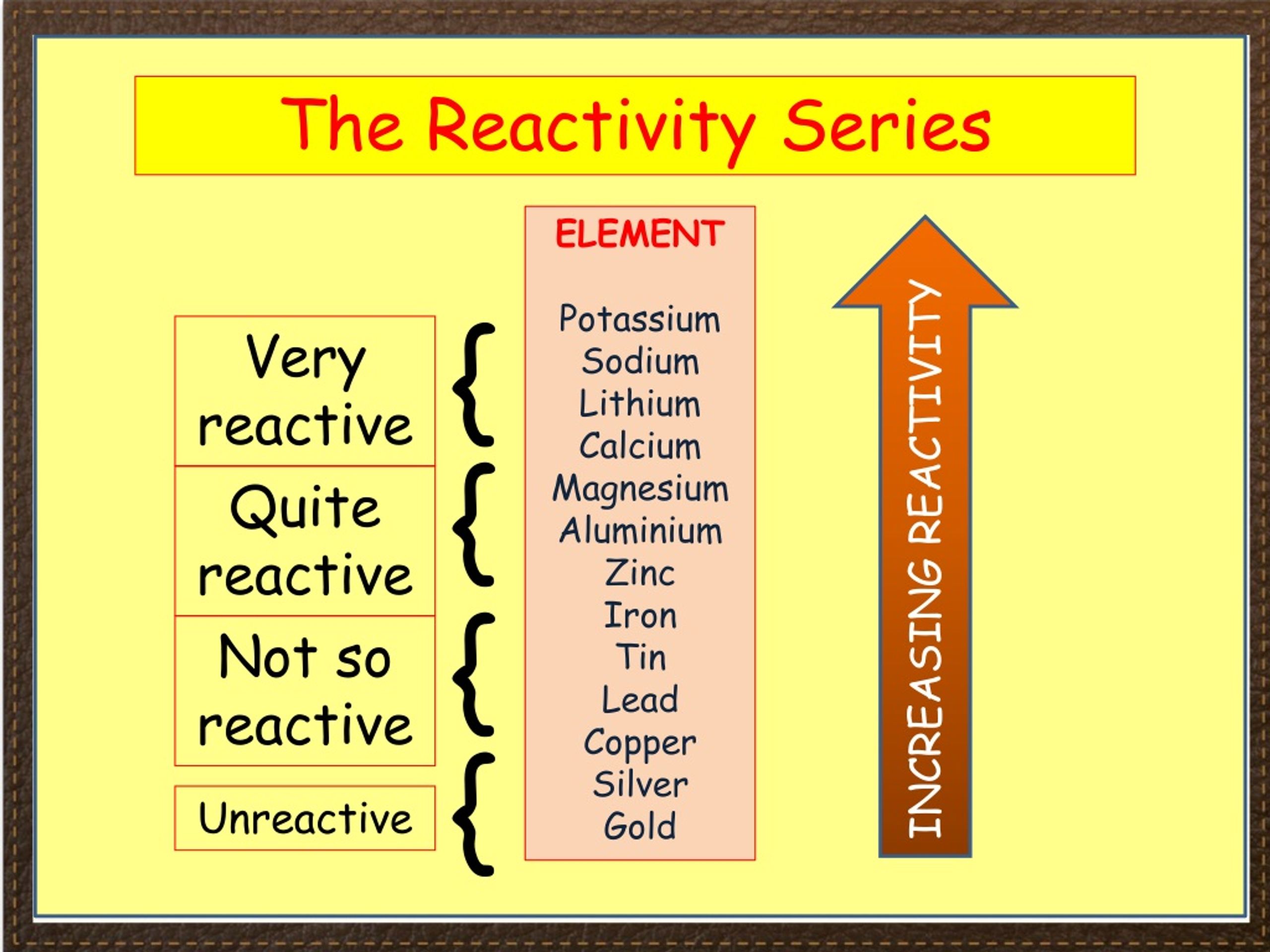
In bioinorganic (and inorganic reactions), there are only a few metals that are really useful for redox.


 0 kommentar(er)
0 kommentar(er)
